Aerobid Prescribing Information
Package insert / product label
Generic name: flunisolide
Dosage form: Inhaler System
Drug class: Inhaled corticosteroids
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Aerobid Description
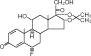
Flunisolide, the active component of AEROBID Inhaler System, is an anti-inflammatory steroid having the chemical name 6α-fluoro-11β, 16α, 17, 21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic-16, 17-acetal with acetone. It has the following structure:
Flunisolide is a white to creamy white crystalline powder with a molecular weight of 434.49. It is soluble in acetone, sparingly soluble in chloroform, slightly soluble in methanol, and practically insoluble in water. it has a melting point of about 245°C.
AEROBID Inhaler is delivered in a metered-dose aerosol system containing a microcrystalline suspension of flunisolide as the hemihydrate in propellants (trichloromonofluoromethane, dichlorodifluoromethane and dichlorotetrafluoroethane) with sorbitan trioleate as a dispersing agent. AEROBID-M also contains menthol as a flavoring agent. Each activation delivers approximately 250 mcg of flunisolide to the patient. One AEROBID Inhaler System is designed to deliver at least 100 metered inhalations.
Aerobid - Clinical Pharmacology
Flunisolide has demonstrated marked anti-inflammatory and anti-allergic activity in classical test systems. It is a corticosteroid that is several hundred times more potent in animal anti-inflammatory assays than the Cortisol standard. The molar dose of each activation of flunisolide in this preparation is approximately 2.5 to 7 times that of comparable inhaled corticosteroid products marketed for the same indication. The dose of flunisolide delivered per activation in this preparation is 10 times that per activation of Nasalide® (flunisolide) nasal solution. Clinical studies have shown therapeutic activity on bronchial mucosa with minimal evidence of systemic activity at recommended doses.
After oral inhalation of 1 mg flunisolide, total systemic availability was 40%. The flunisolide that is swallowed is rapidly and extensively converted to the 6β-OH metabolite and to water-soluble conjugates during the first pass through the liver. This offers a metabolic explanation for the low systemic activity of oral flunisolide itself since the metabolite has the low corticosteroid potency (on the order of the Cortisol standard). The inhaled flunisolide absorbed through the bronchial tree is converted to the same metabolites. Repeated inhalation of 2.0 mg of flunisolide per day (the maximum recommended dose) for 14 days did not show accumulation of the drug in plasma. The plasma half-life of flunisolide is approximately 1.8 hours.
The following observations relevant to systemic absorption were made in clinical studies. In one uncontrolled study a statistically significant decrease in responsiveness to metyrapone was noted in 15 adult steroid-independent patients treated with 2.0 mg of flunisolide per day (the maximum recommended dose) for 3 months. A small but statistically significant drop in eosinophils from 11.5% to 7.4% of total circulating leucocytes was noted in another study in children who were not taking oral corticosteroids simultaneously. A 5% incidence of menstrual disturbances was reported during open studies, in which there were no control groups for comparison.
Aerosol administration of flunisolide 2.0 mg twice daily for one week to 6 healthy male subjects revealed neither suppression of adrenal function as measured by early morning cortisol levels nor impairment of HPA axis function as determined by insulin hypoglycemia tests.
Controlled clinical studies have included over 500 patients with asthma, among them 150 children age 6 and over. More than 120 patients have been treated in open trials for two years or more. No significant adrenal suppression attributed to flunisolide was seen in these studies.
Significant decreases of systemic steroid dosages have been possible in flunisolide-treated patients. Recommended doses of flunisolide appear to be the therapeutic equivalent of an average of 10 mg/day of oral prednisone. Asthma patients have had further symptomatic improvement with flunisolide treatment even while reducing concomitant medication.
Related/similar drugs
prednisone, Breo Ellipta, Xopenex, Xolair, Dulera, Atrovent
Indications and Usage for Aerobid
AEROBID (flunisolide) Inhaler is indicated in the maintenance treatment of asthma as prophylactic therapy. AEROBID is also indicated for asthma patients who require systemic corticosteroid administration, where adding AEROBID may reduce or eliminate the need for the systemic corticosteroids.
AEROBID Inhaler is NOT indicated for the relief of acute bronchospasm.
Contraindications
AEROBID (flunisolide) Inhaler is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required. Hypersensitivity to any of the ingredients of this preparation contraindicates its use.
Warnings
Particular care is needed in patients who are transferred from systemically active corticosteroids to AEROBID Inhaler because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to aerosol corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery or infections, particularly gastroenteritis. Although AEROBID Inhaler may provide control of asthmatic symptoms during these episodes, it does NOT provide the systemic steroid that is necessary for coping with these emergencies. During periods of stress or a severe asthmatic attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume systemic steroids (in large doses) immediately and to contact their physician for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic steroids during periods of stress or a severe asthma attack. To assess the risk of adrenal insufficiency in emergency situations, routine tests of adrenal cortical function, including measurement of early morning resting cortisol levels, should be performed periodically in all patients. An early morning resting cortisol level may be accepted as normal if it falls at or near the normal mean level.
Localized infections with Candida albicans or Aspergillus niger have occurred in the mouth and pharynx and occasionally in the larynx. Positive cultures for oral Candida may be present in up to 34% of patients. Although the frequency of clinically apparent infection is considerably lower, these infections may require treatment with appropriate antifungal therapy or discontinuance of treatment with AEROBID Inhaler.
AEROBID Inhaler is not to be regarded as a bronchodilator and is not indicated for relief of bronchospasm.
Patients should be instructed to contact their physician immediately when episodes of asthma that are not responsive to bronchodilators occur during the course of treatment. During such episodes, patients may require therapy with systemic corticosteroids. Theoretically, the use of inhaled corticosteroids with alternate day prednisone systemic treatment should be accompanied by more HPA suppression than a therapeutically equivalent regimen of either alone.
Transfer of patients from systemic steroid therapy to AEROBID Inhaler may unmask allergic conditions previously suppressed by the systemic steroid therapy, e.g. rhinitis, conjunctivitis, and eczema.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids. In such children or adults who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chicken pox develops, treatment with antiviral agents may be considered.
Precautions
General: Because of the relatively high molar dose of flunisolide per activation in this preparation, and because of the evidence suggesting higher levels of systemic absorption with flunisolide than with other comparable inhaled corticosteroids (see CLINICAL PHARMACOLOGY section), patients treated with AEROBID (flunisolide) should be observed carefully for any evidence of systemic corticosteroid effect, including suppression of bone growth in children. Particular care should be taken in observing patients post-operatively or during periods of stress for evidence of a decrease in adrenal function. During withdrawal from oral steroids, some patients may experience symptoms of systemically active steroid withdrawal, e.g. joint and/or muscular pain, lassitude and depression, despite maintenance or even improvement of respiratory function. (See DOSAGE AND ADMINISTRATION for details.)
In responsive patients, flunisolide may permit control of asthmatic symptoms without suppression of HPA function. Since flunisolide is absorbed into the circulation and can be systemically active, the beneficial effects of AEROBID Inhaler in minimizing or preventing HPA dysfunction may be expected only when recommended dosages are not exceeded.
The long-term local and systemic effects of AEROBID (flunisolide) in human subjects are still not fully known. In particular, the effects resulting from chronic use of AEROBID on developmental or immunologic processes in the mouth, pharynx, trachea, and lung are unknown.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, parasitic or viral infections; or ocular herpes simplex.
Pulmonary infiltrates with eosinophilia may occur in patients on AEROBID Inhaler therapy. Although it is possible that in some patients this state may become manifest because of systemic steroid withdrawal when inhalational steroids are administered, a causative role for the drug and/or its vehicle cannot be ruled out.
Patient Counseling Information
Since the relief from AEROBID Inhaler depends on its regular use and on proper inhalation technique, patients must be instructed to take inhalations at regular intervals. They should also be instructed in the correct method of use. (See Patient Instruction Leaflet.)
Patients whose systemic corticosteroids have been reduced or withdrawn should be instructed to carry a warning card indicating they may need supplemental systemic steroids during periods of stress or a severe asthmatic attack that is not responsive to bronchodilators.
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
An illustrated leaflet of patient instructions for proper use accompanies each AEROBID Inhaler System.
CONTENTS UNDER PRESSURE
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F (49°C) may cause container to explode. Never throw container into fire or incinerator. Keep out of reach of children.
Carcinogenesis: Long-term studies were conducted in mice and rats using oral administration to evaluate the carcinogenic potential of the drug. There was an increase in the incidence of pulmonary adenomas in mice, but not in rats.
Female rats receiving the highest oral dose had an increased incidence of mammary adenocarcinoma compared to control rats. An increased incidence of this tumor type has been reported for other corticosteroids.
Impairment of Fertility: Female rats receiving high doses of flunisolide (200 mcg/kg/ day) showed some evidence of impaired fertility. Reproductive performance in the low- (8 mcg/kg/day) and mid-dose (40 mcg/kg/day) groups was comparable to controls.
Pregnancy: Pregnancy Category C. As with other corticosteroids, flunisolide has been shown to be teratogenic in rabbits and rats at doses of 40 and 200 mcg/kg/day respectively. It was also fetotoxic in these animal reproductive studies. There are no adequate and well-controlled studies in pregnant women. Flunisolide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because other corticosteroids are excreted in human milk, caution should be exercised when flunisolide is administered to nursing women.
Pediatric Use: Safety and effectiveness have not been established in children below the age of 6. Oral corticoids have been shown to cause growth suppression in children and adolescents, particularly with higher doses over extended periods. If a child or adolescent on any corticoid appears to have growth suppression, the possibility that they are particularly sensitive to this effect of steroids should be considered.
Adverse Reactions/Side Effects
Adverse events reported in controlled clinical trials and long-term open studies in 514 patients treated with AEROBID (flunisolide) are described below. Of those patients, 463 were treated for 3 months or longer, 407 for 6 months or longer, 287 for 1 year or longer, and 122 for 2 years or longer.
Musculoskeletal reactions were reported in 35% of steroid-dependent patients in whom the dose of oral steroid was being tapered. This is a well-known effect of steroid withdrawal.
Incidence 10% or greater:
- Gastrointestinal: diarrhea (10%), nausea and/or vomiting (25%), upset stomach (10%)
- General: flu (10%)
- Mouth and Throat: sore throat (20%)
- Nervous System: headache (25%)
- Respiratory: cold symptoms (15%), nasal congestion (15%), upper respiratory infection
- (25%)
- Special Senses: unpleasant taste (10%)
Incidence 3-9%
- Cardiovascular: palpitations
- Gastrointestinal: abdominal pain, heartburn
- General: chest pain, decreased appetite, edema, fever
- Mouth and Throat: Candida infection
- Nervous System: dizziness, irritability, nervousness, shakiness
- Reproductive: menstrual disturbances
- Respiratory: chest congestion, cough*, hoarseness, rhinitis, runny nose, sinus congestion,
- sinus drainage, sinus infection, sinusitis, sneezing, sputum, wheezing1
- Skin: eczema, itching (pruritus), rash
- Special Senses: ear infection, loss of smell or taste
Incidence 1-3%
- General: chills, increased appetite and weight gain, malaise, peripheral edema, sweating,
- weakness
- Cardiovascular: hypertension, tachycardia
- Gastrointestinal: constipation, dyspepsia, gas
- Hemic/Lymph: capillary fragility, enlarged lymph nodes
- Mouth and Throat: dry throat, glossitis, mouth irritation, pharyngitis, phlegm, throat irritation
- Nervous System: anxiety, depression, faintness, fatigue, hyperactivity, hypoactivity,
- insomnia, moodiness, numbness, vertigo
- Respiratory: bronchitis, chest tightness*, dyspnea, epistaxis, head stuffiness, laryngitis,
- nasal irritation, pleurisy, pneumonia, sinus discomfort
- Skin: acne, hives or urticaria
- Special Senses: blurred vision, earache, eye discomfort, eye infection
Incidence less than 1%, judged by investigators as possibly or probably drug related:
- abdominal fullness, shortness of breath.
- 1
- The incidences as shown of cough, wheezing, and chest tightness were judged by investigators to be possibly or probably drug related. In placebo-controlled trials, the overall incidences of these adverse events (regardless of investigators' judgement of drug relationship) were similar for drug and placebo-treated groups. They may be related to the vehicle or delivery system.
Aerobid Dosage and Administration
The AEROBID (flunisolide) Inhaler System is for oral inhalation only. Adults: The recommended starting dose is 2 inhalations twice daily, morning and evening, for a total daily dose of 1 mg. The maximum daily dose should not exceed 4 inhalations twice a day for a total daily dose of 2 mg. When the drug is used chronically at 2 mg/day, patients should be monitored periodically for effects on the hypothalamic-pituitary-adrenal (HPA) axis.
Pediatric Patients: For children and adolescents 6-15 years of age, two inhalations may be administered twice daily for a total daily dose of 1 mg. Higher doses have not been studied. Insufficient information is available to warrant use in children under age 6. With chronic use, pediatric patients should be monitored for growth as well as for effects on the HPA axis.
Rinsing the mouth after inhalation is advised.
Different considerations must be given to the following groups of patients in order to obtain the full therapeutic benefit ofAEROBID (flunisolide) Inhaler.
Patients Not Receiving Systemic Corticosteroids:
Patients who require maintenance therapy of their asthma may benefit from treatment with AEROBID at the doses recommended above. In patients who respond to AEROBID, improvement in pulmonary function is usually apparent within one to four weeks after the start of therapy. Once the desired effect is achieved, consideration should be given to tapering to the lowest effective dose.
Patients Maintained on Systemic Corticosteroids:
Clinical studies have shown that AEROBID may be effective in the management of asthmatics dependent or maintained on systemic corticosteroids and may permit replacement or significant reduction in the dosage of systemic corticosteroids.
The patient's asthma should be reasonably stable before treatment with AEROBID is started. Initially, AEROBID should be used concurrently with the patient's usual maintenance dose of systemic corticosteroid. After approximately one week, gradual withdrawal of the systemic corticosteroid is started by reducing the daily or alternate daily dose. Reductions may be made after an interval of one or two weeks, depending on the response of the patient. As low rate of withdrawal is strongly recommended. Generally, these decrements should not exceed 2.5 mg of prednisone or its equivalent. During withdrawal, some patients may experience symptoms of systemic corticosteroid withdrawal; e.g. joint and/or muscular pain, lassitude and depression, despite maintenance or even improvement in pulmonary function. Such patients should be encouraged to continue with the inhaler but should be monitored for objective signs of adrenal insufficiency. If evidence of adrenal insufficiency occurs, the systemic corticosteroid doses should be increased temporarily and thereafter withdrawal should continue more slowly.
During periods of stress or a severe asthma attack, transfer patients may require supplementary treatment with systemic corticosteroids.
How is Aerobid supplied
AEROBID (flunisolide) Inhaler Systems are available in canisters of 100 metered inhalations.
NDC 0456-0672-99 AEROBID
NDC 0456-0670-99 AEROBID-M
"Note: The indented statement below is required by the Federal government's Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFC's)."
WARNING: Contains trichloromonofluoromethane, dichlorodifluoromethane and dichlorotetrafluoroethane, substances which harm public health and environment by destroying ozone in the upper atmosphere.
"A notice similar to the above WARNING has been placed in the information for the patient of this product pursuant to EPA regulations."
mfd for
FOREST PHARMACEUTICALS, INC.
St. Louis, MO 63045
mfd by
3M Pharmaceuticals
St. Paul, MN
Rev 3/02
624200
How to use your
AEROBID®AEROBID®-M
(flunisolide)
Inhaler System
DIRECTIONS FOR USE:
Before using your new AEROBID Inhaler System, it is important that you read over the following simple instructions and familiarize yourself with the inhaler and its metal cartridge.
As your doctor has probably told you, the AEROBID Inhaler System must be used for a few days before it begins working, and then should be used regularly to help reduce the frequency and severity of your asthma attacks. It is not a bronchodilator and will not provide relief during an actual asthmatic attack, but it can cut down the number of bad attacks if used regularly every day.
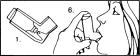
- Before the first use, place the AEROBID metal cartridge inside the plastic container as shown.
- Shake the inhaler system before each inhalation.
- Before each use, remove dustcap and inspect mouthpiece for foreign objects.
- Replace dustcap after each use.
- Breathe out as completely as possible.
- Hold the inhaler system upright and put plastic mouthpiece in your mouth as shown, being sure to close your lips tightly around the mouthpiece.
- Breathe in slowly through your mouth. At the same time firmly press down on the metal cartridge with your index finger.
- Hold your breath as long as you can.
- While holding your breath, stop pressing on the cartridge and remove mouthpiece from your mouth.
- If your doctor has prescribed two or more inhalations at each use, wait a minute to allow pressure to build up again in the metal canister, then repeat steps two through nine (2-9). Be sure to shake the inhaler system again before each inhalation.
- After the prescribed number of inhalations, rinse out your mouth thoroughly with water.
- Clean the inhaler system every few days. To do so, remove the metal cartridge, then rinse the plastic inhaler and cap with briskly running warm water. Dry thoroughly. Replace the cartridge and cap.
NOTE: If your mouth becomes sore or develops a rash, be sure to mention this to your doctor, but do not stop using your inhaler system unless he tells you.
WARNING: The contents of the metal cartridge are under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperature above 120°F (49°C) may cause cartridge to explode. Never throw cartridge into fire or incinerator. Use by children should always be supervised by an adult.
mfd by
3M Pharmaceuticals, Inc. St. Paul, MN
For:
FOREST PHARMACEUTICALS, INC.
SUBSIDIARY OF FOREST LABORATORIES, INC.
ST. LOUIS, MISSOURI 63045
| AEROBID
flunisolide aerosol, metered |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AEROBID-M
flunisolide aerosol, metered |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - FOREST PHARMACEUTICALS, INC. |
More about Aerobid (flunisolide)
- Check interactions
- Compare alternatives
- Reviews (1)
- Side effects
- Dosage information
- During pregnancy
- Drug class: inhaled corticosteroids
- Breastfeeding